Storing hydrogen fuel in salts—a step toward ‘cleaner’ energy production
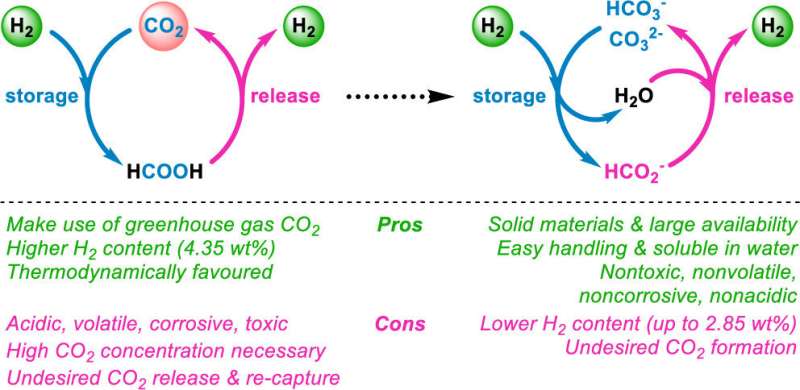
Hydrogen gas could someday replace fossil fuels as a “clean” energy source, producing only water and energy. However, handling large quantities of gaseous hydrogen is cumbersome, and converting it to a liquid requires vessels that can withstand extremely high pressures. Now, researchers reporting in ACS Central Science have developed a method to store and release highly pure hydrogen with salts in the presence of amino acids.
The reversible storage of hydrogen in solid salts has emerged as one potential way to make the fuel easier to transport and handle, but the reactions to do this require precious metals as catalysts and may produce carbon dioxide as an unwanted byproduct. So, Henrik Junge, Matthias Beller and colleagues developed effective storage-release systems with both bicarbonate and carbonate salts, as well as manganese, which is a more widely available metal catalyst.
The researchers found that converting bicarbonate and hydrogen into formate, and vice versa, was most effective with potassium salts, a manganese-based catalyst and lysine—an amino acid that acted as an additional promoter and reacted with carbon dioxide to capture it—at reaction temperatures below 200 F. After five storage-release cycles, the reaction system produced hydrogen with a high yield (80%) and purity (99%).
The team also showed that carbonate salts and glutamic acid can be part of the reusable storage-release system with hydrogen yields up to 94%. This technique paves the way for large-scale hydrogen storage in solids, the researchers say.
A system for the reversible hydrogenation of carbon dioxide into formic acid
Duo Wei et al, Manganese Promoted (Bi)carbonate Hydrogenation and Formate Dehydrogenation: Toward a Circular Carbon and Hydrogen Economy, ACS Central Science (2022). DOI: 10.1021/acscentsci.2c00723
Citation:
Storing hydrogen fuel in salts—a step toward ‘cleaner’ energy production (2022, October 19)
retrieved 19 October 2022
from https://techxplore.com/news/2022-10-hydrogen-fuel-saltsa-cleaner-energy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
