A heteropoly acid negolyte that could enhance the performance of aqueous redox flow batteries at low temperatures
In recent years, engineers have been developing a wide range of new battery technologies and solutions. One of these are redox flow batteries, devices that can store electrochemical energy by converting chemical energy into electrical energy via the reversible oxidation and reduction of fluids.
These batteries could have many valuable applications. Most notably, however, they could enable the reliable and cost-effective storage of the energy generated by sustainable energy solutions, such as solar panels or wind turbines.
Aqueous redox flow batteries (ARFBs) are a specific type of redox flow batteries in which electrical energy is stored in two redox-active species with different redox potentials, which are dissolved in electrolyte tanks. This unique design can improve their safety, while also enabling the decoupling of energy and power in the devices. Despite their advantages, so far ARFBs could not be used at low temperatures, due to the limited solubility of the redox-active materials contained in them, as well as other issues.
Researchers at the Chinese University of Hong Kong have recently developed a new heteropoly acid negolyte, a component for ARFBs containing a type of acid that combines hydrogen and oxygen with specific metals or non-metals. This negolyte, presented in a paper published in Nature Energy, could help to improve the performance of ARFBs at low temperatures.
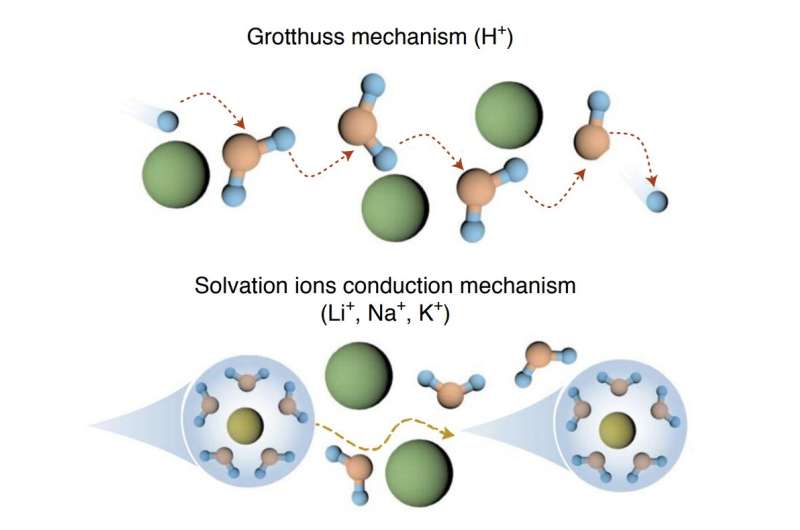
“Operating aqueous redox flow batteries (ARFBs) at low temperatures is prohibited by limited solubility of redox-active materials, freezing electrolytes and sluggish reaction kinetics,” the researchers wrote in their paper. “We report a multi-electron heteropoly acid (H6P2W18O62, HPOM) negolyte that enables high-performance ARFBs at low temperatures.”
The heteropoly acid negolyte introduced by the researchers, dubbed HPOM, has a unique design that increases its solubility. In contrast with previously used negolytes, therefore, it enables these batteries to perform well at low temperatures.
“The proton (H+) in HPOM warrants a much higher solubility of polyoxometalates (POMs) (0.74 M at 25 °C and 0.5 M at −20 °C) compared with other cations (Li+/Na+/K+) owing to the strong solvation shell of H+ preventing precipitation,” the researchers explained in their paper.
The researchers evaluated their heteropoly acid negolytes in a series of experiments and found that it exhibits an exceptionally high electron solubility, a high conductivity, a low freezing point and high redox kinetics. These qualities are all highly advantageous for the fabrication of ARBSs with a high power density that can operate at low temperatures.
“Using a 0.5 M HPOM electrolyte, the ARFBs demonstrate power density (282.4 mW cm−2 ) and stability (79.6 Ah l−1 negolyte at 160 mA cm−2 over 1,200 h without decay) at −20 °C, showing promising application potential under cold weather conditions,” the researchers wrote in their paper.
In the future, the heteropoly acid negolyte introduced by this team of researchers could be used to create new ARFBs that can also store energy at low temperatures. This could facilitate the storage of energy produced by sustainable energy sources in places with extreme weather conditions, characterized by particularly low temperatures.
Fei Ai et al, Heteropoly acid negolytes for high-power-density aqueous redox flow batteries at low temperatures, Nature Energy (2022). DOI: 10.1038/s41560-022-01011-y
© 2022 Science X Network
Citation:
A heteropoly acid negolyte that could enhance the performance of aqueous redox flow batteries at low temperatures (2022, May 27)
retrieved 27 May 2022
from https://techxplore.com/news/2022-05-heteropoly-acid-negolyte-aqueous-redox.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
