Chemical crossover accelerates degradation of electrode in high energy density rechargeable lithium–oxygen batteries
The National Institute for Materials Science (NIMS), SoftBank Corp. and Ohara Inc. have jointly identified the detailed mechanisms behind the degradation of high-energy-density lithium-oxygen batteries.
Analytical studies using a combination of advanced techniques reveals that lithium negative electrode severely degraded when associated with the progress cycle, resulting in the short cycle life of lithium-oxygen batteries. Through use of a lightweight protective layer for lithium electrodes, researchers largely extended the cycle life of lithium-oxygen batteries without diminishing their high energy density (measured in Wh/kg).
Their findings are published in the journal Advanced Energy Materials.
Lithium-oxygen batteries have the potential to be the ultimate rechargeable batteries: they are lightweight and high capacity, with theoretical energy densities per unit weight several times that of currently available lithium ion batteries. Because of these potential advantages, there is growing attention for use of this battery system for a wide range of technologies, such as drones, electric vehicles and household electricity storage systems.
NIMS has been carrying out basic research on lithium-oxygen batteries. This program has the goal of accelerating large-capacity rechargeable battery R&D.
In 2018, NIMS and SoftBank co-founded their Advanced Technologies Development Center to conduct research with the aim of putting lithium-oxygen batteries into practical use in mobile phone base stations, the Internet of Things (IoT), HAPS (high altitude platform stations) and other technologies. In 2021, the Center succeeded in developing a lithium-oxygen battery with an energy density of nearly 500 Wh/kg, greatly surpassing the energy densities of previously developed lithium-oxygen batteries.
However, the life of this battery was only 10 charge/discharge cycles or less, which required attention before it could be put into practical use.
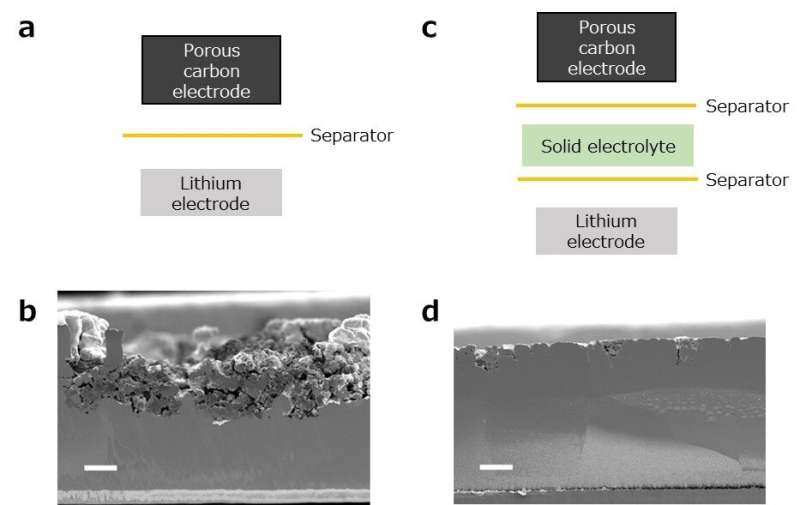
Using a combination of various previously developed advanced analytical techniques, the research team found that a lithium-oxygen battery’s metallic lithium negative electrode degrades rapidly while being subjected to charge/discharge cycles, causing significant overpotential in the battery and shortening its cycle life.
This result contradicts the conventional belief that cycle-life-reducing overpotential is induced by electrochemical reactions taking place in the oxygen positive electrode. To prevent the degradation of the metallic lithium negative electrode, the research team also developed a light and flexible solid electrolyte 6 μm in thickness and integrated it into a lithium-oxygen battery as a protective layer. As a result, the battery’s cycle life was significantly extended without compromising its high energy density per unit weight.
The research team hopes to expedite the development of practical lithium-oxygen batteries at the NIMS-SoftBank Advanced Technologies Development Center by developing new battery materials and incorporating them into the batteries.
More information:
Shoichi Matsuda et al, Chemical Crossover Accelerates Degradation of Lithium Electrode in High Energy Density Rechargeable Lithium–Oxygen Batteries, Advanced Energy Materials (2023). DOI: 10.1002/aenm.202203062
Citation:
Chemical crossover accelerates degradation of electrode in high energy density rechargeable lithium–oxygen batteries (2023, March 16)
retrieved 16 March 2023
from https://techxplore.com/news/2023-03-chemical-crossover-degradation-electrode-high.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.

