Impeding the impedance: Research reveals how to design a better next-generation lithium-ion battery
The newest generation of lithium-ion batteries now under development promises a revolution in powering cell phones, electric vehicles, laptops and myriad other devices. Featuring all solid-state, nonflammable components, the new batteries are lighter, hold their charge longer, recharge faster and are safer to use than conventional lithium-ion batteries, which contain a gel that can catch on fire.
However, like all batteries, solid-state lithium-ion batteries have a drawback: Due to electro-chemical interactions, impedance—the AC analog of DC electrical resistance—can build up within the batteries, limiting the flow of electric current. Researchers at the National Institute of Standards and Technology (NIST) and their colleagues have now pinpointed the location where most of this buildup occurs. In so doing, the team has suggested a simple redesign that could dramatically limit the buildup of impedance, enabling the batteries to fulfill their role as the next-generation power source.
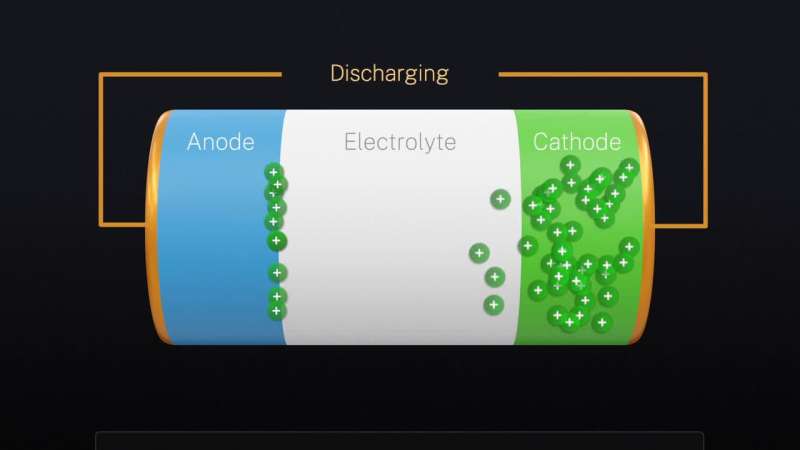
A lithium-ion battery consists of two sheetlike terminals, the anode (negative terminal) and the cathode (positive terminal), separated by an ion-conducting medium called the electrolyte. (The electrolyte is a gel in the case of ordinary lithium-ion batteries, a solid in the solid-state version.) During discharging, lithium ions flow from the anode through the electrolyte to the cathode, forcing electrons to move around an outside circuit and generate the electric current that powers devices.
Impedance typically arises at the interface between either of the two electrodes and the electrolyte. But finding the exact location requires knowledge of both the distribution of lithium ions and the difference in voltage at each interface.
Previous studies by other teams could not definitively locate the problem area because the tool they used averaged impedance over the entire battery rather than measuring it at individual sites within the device. The NIST team, which includes collaborators from the Sandia National Laboratory in Livermore, California, the Naval Research Laboratory in Washington, D.C. and several universities, used two complementary methods to study impedance on the nanoscale in a solid-state lithium-ion battery.
One method, Kelvin probe force microscopy, uses the sharp tip of an atomic force microscope hovering over the different layers of an open battery to image the distribution of voltage on each surface. The probe revealed that the largest drop in voltage within the battery occurred at the electrolyte/anode interface, indicating this was a region of high impedance. (If the entire battery had low impedance, the internal voltage drop would vary gradually and smoothly from place to place inside the cell.)
The second method, neutron depth profiling, uses a beam of low-energy neutrons generated at the NIST Center for Neutron Research to probe the nanoscale distribution and concentration of lithium. Because neutron depth profiling does not harm the battery, the researchers were able to employ the technique while the battery was running.
When low-energy neutrons from the beam were absorbed by lithium in the battery, they produced energetic charged particles, alpha (4He) and tritium (3H). The number of these charged particles generated and the energy they retain after passing through the layers of the battery indicate the concentration of lithium ions at different places in the battery.
The measurements revealed that the main site where the lithium ions had piled up, diminishing the flow of electric current, occurred at the boundary between the electrolyte and the anode—the same site at which the Kelvin probe force microscopy had detected the largest voltage drop.
Taken together, the results of the Kelvin probe force microscopy and neutron depth profiling techniques unequivocally demonstrated that most of the impedance arises at the electrolyte/anode interface, said team member Evgheni Strelcov of NIST and the University of Maryland NanoCenter in College Park.
Strelcov and other researchers, including Jamie Weaver, Joseph Dura, Andrei Kolmakov and Nikolai Zhitenev of NIST and their collaborators, reported their findings online October 19 in the journal ACS Energy Letters.
“This work demonstrates that neutron depth profiling, combined with Kelvin probe force microscopy and theoretical modeling, continues to advance our understanding of the inner workings of lithium-ion batteries,” said Weaver.
In analyzing their findings, the scientists concluded that the impedance they found at the interface could be significantly reduced if layers of other material were added in between the anode and the electrolyte. Adding intervening layers that properly adhere to each other would prevent the electrolyte and anode from interacting with each other directly. That’s a benefit because when an electrolyte and the anode are in direct contact, they sometimes form a thin layer of material that impedes the transport of the ions.
“We want to engineer the interfaces so that they have high ion and electron conductivity,” Strelcov said.
New scalable method resolves materials joining in solid-state batteries
Elliot J. Fuller et al, Spatially Resolved Potential and Li-Ion Distributions Reveal Performance-Limiting Regions in Solid-State Batteries, ACS Energy Letters (2021). DOI: 10.1021/acsenergylett.1c01960
Citation:
Impeding the impedance: Research reveals how to design a better next-generation lithium-ion battery (2021, November 16)
retrieved 16 November 2021
from https://techxplore.com/news/2021-11-impeding-impedance-reveals-next-generation-lithium-ion.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.

