Mn-rich phosphate cathodes with better electrochemical performance for Na-ion batteries
A research group led by Prof. Zhao Junmei from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences developed a novel vanadium-poor and manganese-rich phosphate cathode with better electrochemical performance for Na-ion batteries.
The study was published in ACS Energy Letters on Dec. 3.
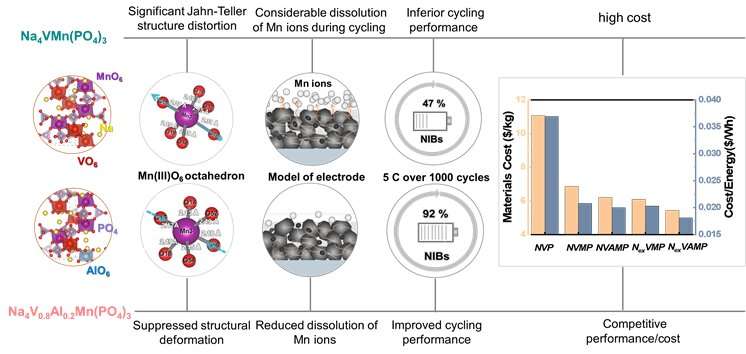
This work addresses the crucial issues on how to effectively suppress the Jahn-Teller distortions of Mn3+, meanwhile minimizing the cost of raw materials and enhancing the electrochemical performance in the Mn-rich phosphate cathodes.
The so called Jahn-Teller distortion means a geometric structure deformation of MnO6 octahedron, resulted from the shrinkage or elongation of the O-Mn-O bond when Mn is in the high-spin Mn3+ oxidation state. Such a structural distortion is usually accompanied by the serious structure degradation and sluggish kinetics, finally leading to the inferior electrochemical performance.
Mn-based phosphate cathodes are promising due to low cost and high voltage, particularly the Mn-rich NASICON structure. Thus, it’s significant to effectively suppress the undesired Mn3+ Jahan-Teller effect in the Mn-rich phosphate cathodes.
In this study, the researchers designed the Al3+ as a stabilizer for selective replacement of V instead of Mn to tune the crystal structure of Mn-based Na4VMn(PO4)3 system. DFT calculation affirmed that the structural distortions and the dissolution of Mn ions were effectively suppressed due to enhanced ionic-covalent character after Al substitution.
The resulting Na4V0.8Al0.2Mn(PO4)3 achieved a capacity retention over 92 percent after cycling 1000 times at 5 C, which was far superior to that (only 47 percent) of Na4VMn(PO4)3 cathode.
Benefiting from the synergistic effects among V, Al and Mn multi-elements, the Na4V0.8Al0.2Mn(PO4)3 exhibited enhanced ion diffusion ability and electronic conductivity. As a result, the Al incorporated Na4V0.8Al0.2Mn(PO4)3 cathode could deliver a superior rate capacity of 84 mA h g-1 at 40 C, even with the loading as high as 5.5 mg/cm2, significantly better than 62 mA h g-1 for Na4VMn(PO4)3.
Furthermore, a Mn-richer Na4.2V0.6Al0.2Mn1.2(PO4)3 was also proposed and developed as a viable candidate for Mn-richer phosphates cathodes.
Compared with Na3V2(PO4)3 and Na4VMn(PO4)3, the raw materials costs of Al incorporated Na4V0.8Al0.2Mn(PO4)3 cathode were reduced by nearly 44 percent and 10 percent, respectively. More impressively, those values for the Al substituted Mn-richer Na4.2V0.6Al0.2Mn1.2(PO4)3 could reach 50 percent and 20 percent, respectively.
Doubling the charging-recharging cycle of lithium batteries
Chunliu Xu et al, Mn-Rich Phosphate Cathodes for Na-Ion Batteries with Superior Rate Performance, ACS Energy Letters (2021). DOI: 10.1021/acsenergylett.1c02107
Citation:
Mn-rich phosphate cathodes with better electrochemical performance for Na-ion batteries (2021, December 15)
retrieved 15 December 2021
from https://techxplore.com/news/2021-12-mn-rich-phosphate-cathodes-electrochemical-na-ion.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.

