Moderna Wants The FDA To Approve Its COVID-19 Shot For This Controversial Age Group
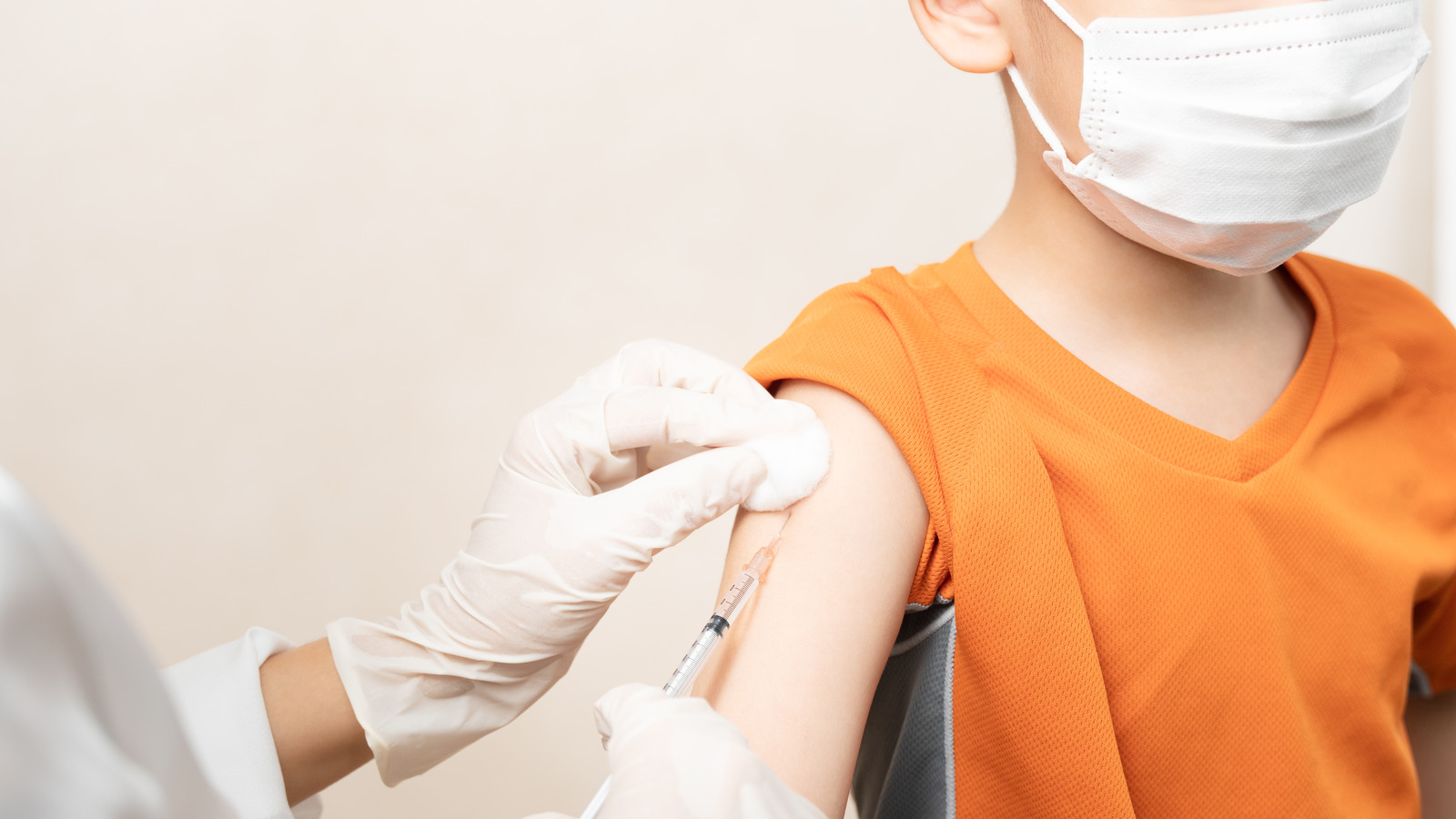
If the vaccine becomes authorized for emergency use by the FDA, the kids who take it will receive much smaller doses than adults. The tested dose included two shots, 25 micrograms each, compared to the 100 micrograms that adults are given up to four times as part of their vaccination series. However, the study shows that these smaller doses are sufficient and effective in preventing severe disease outcomes from developing in younger kids. Of all the 6,700 participants in this part of the KidCOVE study, not a single child developed a severe case of COVID. There was an absence of hospitalization and death, both good indicators that Moderna might be a viable solution for these younger age groups.
As of now, children under 5 years old are the only age group left in the U.S. that is currently not granted access to COVID vaccines. The FDA has promised to move quickly in order to authorize such vaccines once the necessary studies have been completed and all papers filed. Unsurprisingly, Moderna is not the only pharmaceutical giant to work on this type of vaccine for young children — Pfizer has a similar vaccine in the pipeline, too. However, Pfizer’s variant is for kids ages 6 months through 4 years.
Things have been quiet where Pfizer’s vaccine is concerned since the company announced in February 2022 that it will postpone its rolling application for authorization to the FDA. It is still currently awaiting results from the last phase of testing. Unfortunately, recent findings show that a different Pfizer product, the Paxlovid antiviral drug, has been linked with rebounding cases of COVID, which is another thing the FDA will likely need to address in the near future.
For all the latest Games News Click Here
For the latest news and updates, follow us on Google News.
